[객원 에디터 3기 / 장석현 기자] Researchers at the Tokyo Metropolitan University have recently discovered a brain protein called ‘Tau’ with a unique chemical feature that allows it to accumulate within the brain when abnormally tangled and potentially lead to neurodegenerative disorders – tauopathies – such as Alzheimer’s and Pick’s disease.
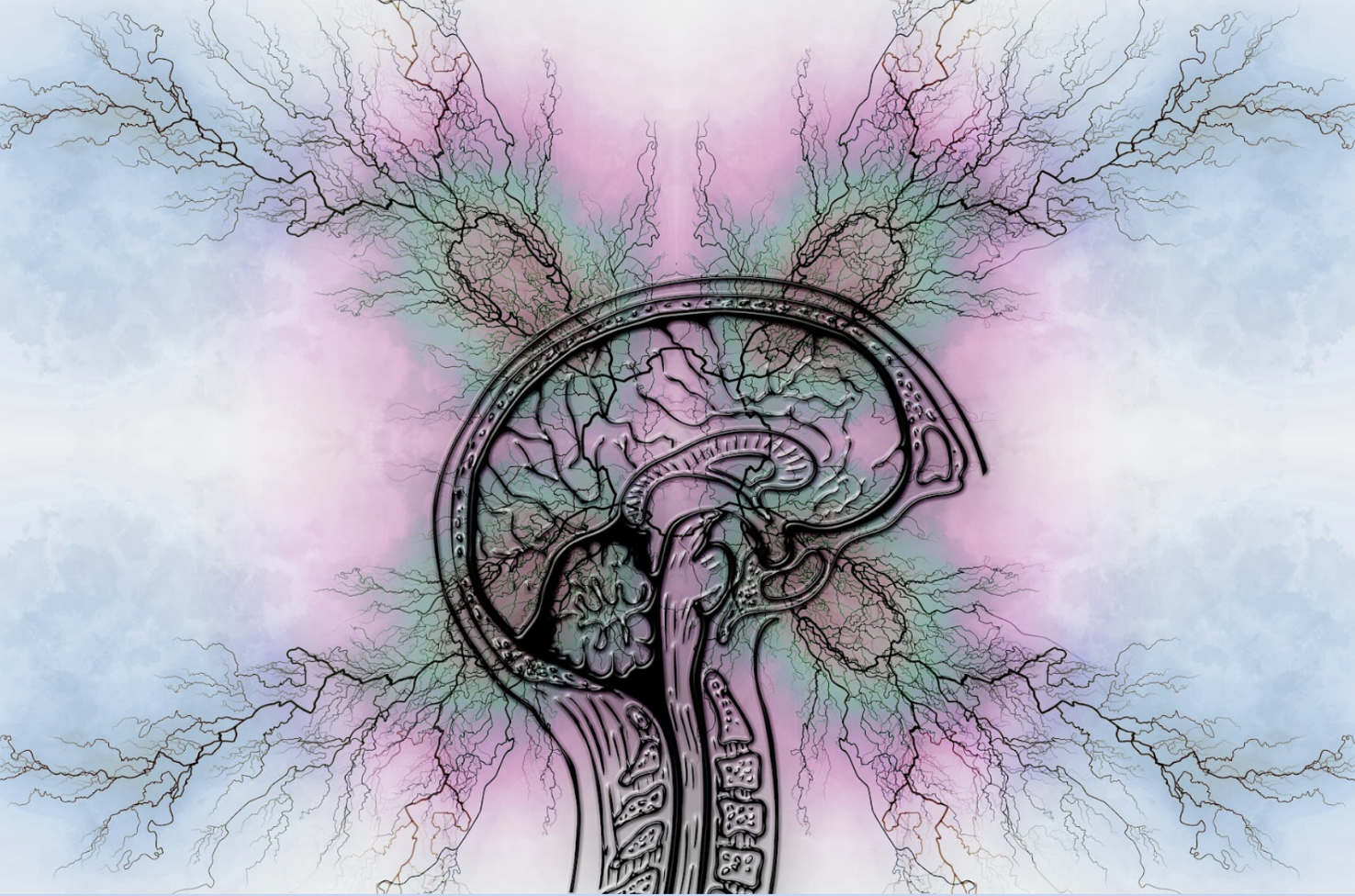
Tau proteins are found inside brain cells called neurons, which have played a predominant role in making up the microtubules inside our brain. Microtubules are fragile cell filaments that play the essential role of ‘rigid highways’ to maintain the Axons – structures that transmit neurotransmitters and chemo electrical signals to allow cellular communications between neurons.
When these Tau proteins accumulate in clumps in the brain, they cause neurofibrillary tangles within the neurons. The Astrocytes and Microglia that serve as debris cleaners cannot respond to Tau due to the speed and continuity of their spread within the brain, resulting in chronic inflammations that eventually cause the neurons to die. In the case of Alzheimer’s disease, considering how Tau accumulation begins in the Hippocampus, the primary brain section responsible for memory and decision-making, explains why tau proteins can potentially trigger Alzheimer’s.
It is an essential scientific advancement to discover the ‘switch’ that transformed the essential Tau proteins into threatening organelles. Luckily, scientists have discovered this during their most recent work using a genetically altered Drosophila fruit fly. When the oxidative stress level of the body increases due to extensive exposures to reactive oxygens like Thiol, it forces the disulfide bonds to form between amino acid residues of a protein known as Cysteines in two specific locations called C291 and C322. The cytesines were oxidized to form these bonds and caused alterations that increased the toxic mutants within Tau.
In other words, examining how increased disulfide bonds within Cysteines led to increased toxicity of Tau means that the team has also pinned down the chemical features that could reverse their toxicity back to normal. The researchers are now hoping for new therapies that can tackle this discovery to be made for many patients suffering from Tauopathies across the globe.
Sources: SciTechDaily, Brightfocus.org, Institut-curie, Drexel.edu